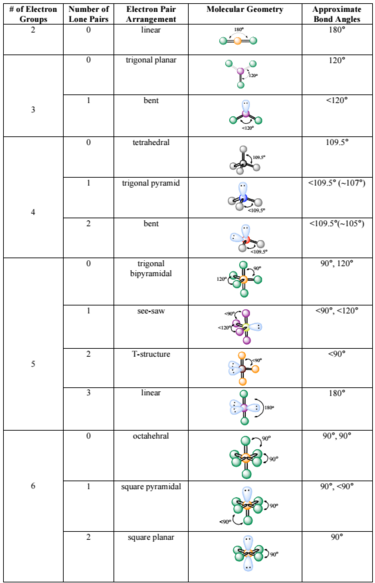
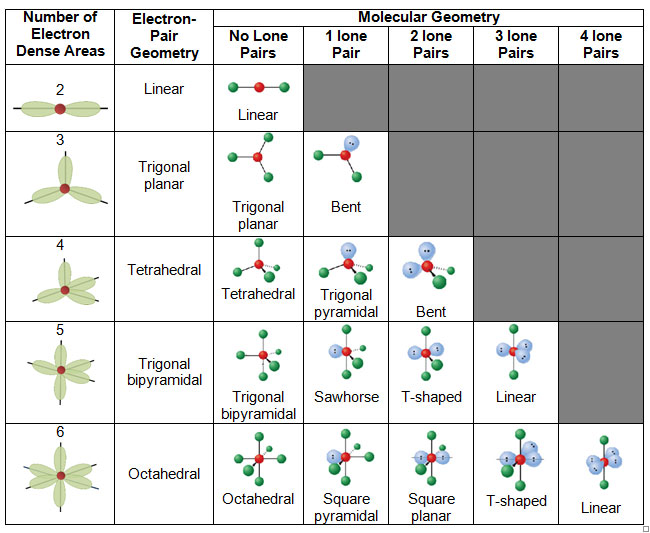
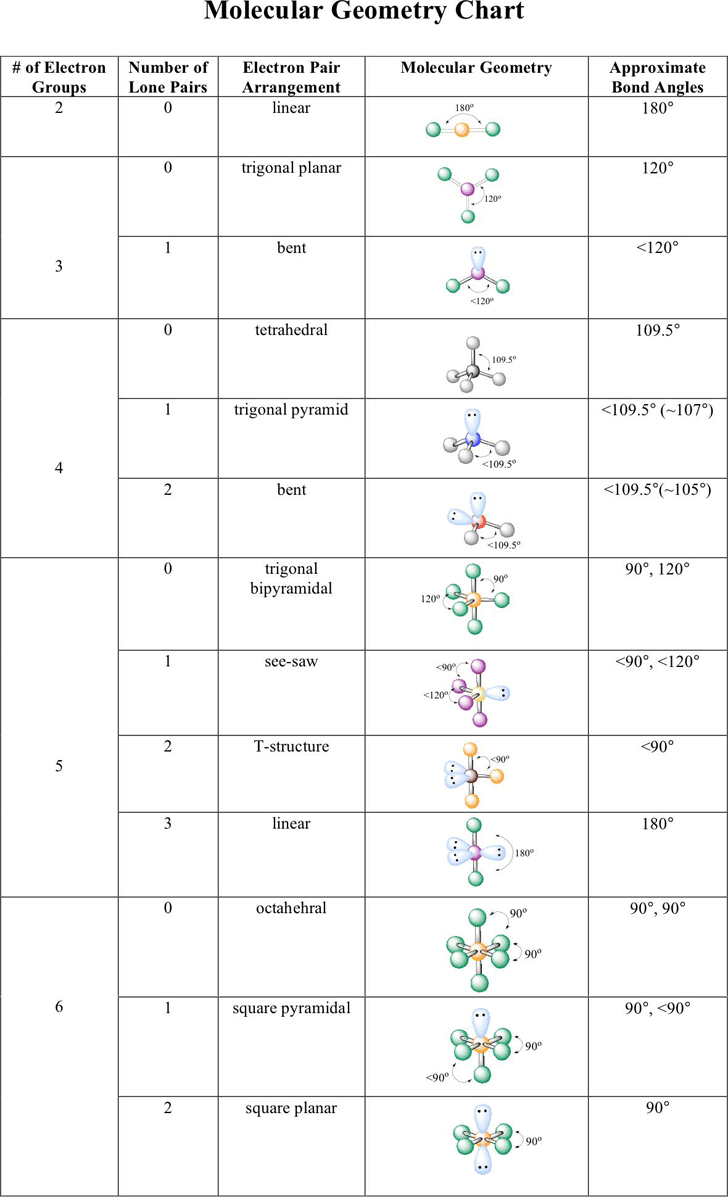
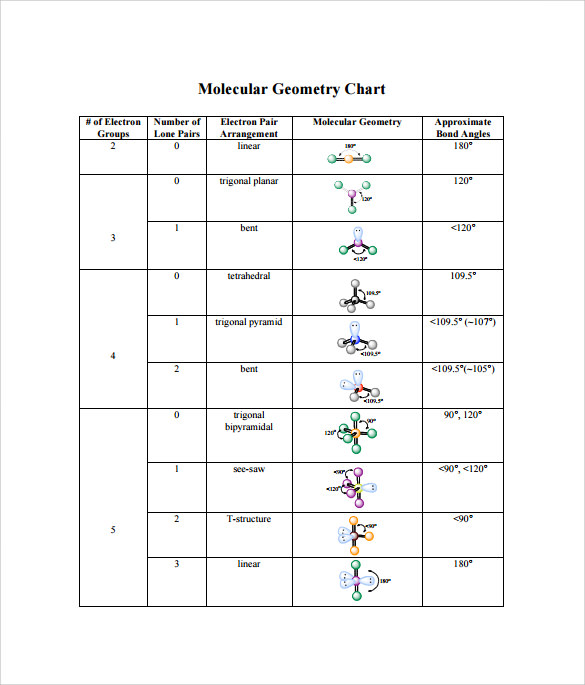
Electron Pair Geometry Chart
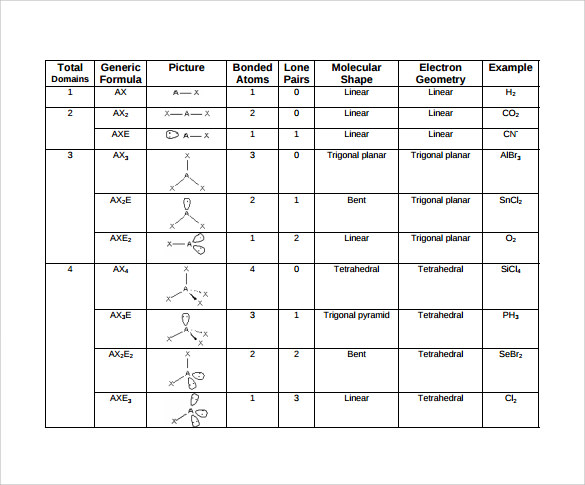
Molecular geometry can be determined from the VSEPR chart.
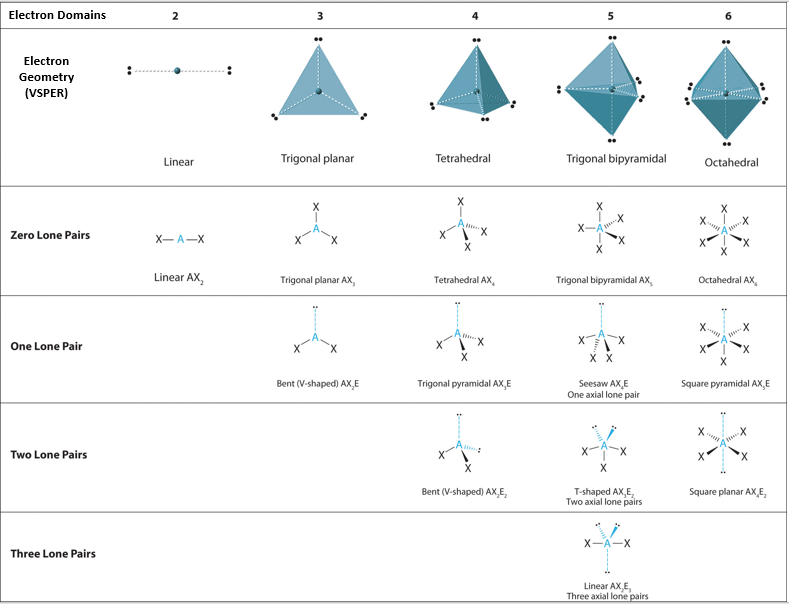
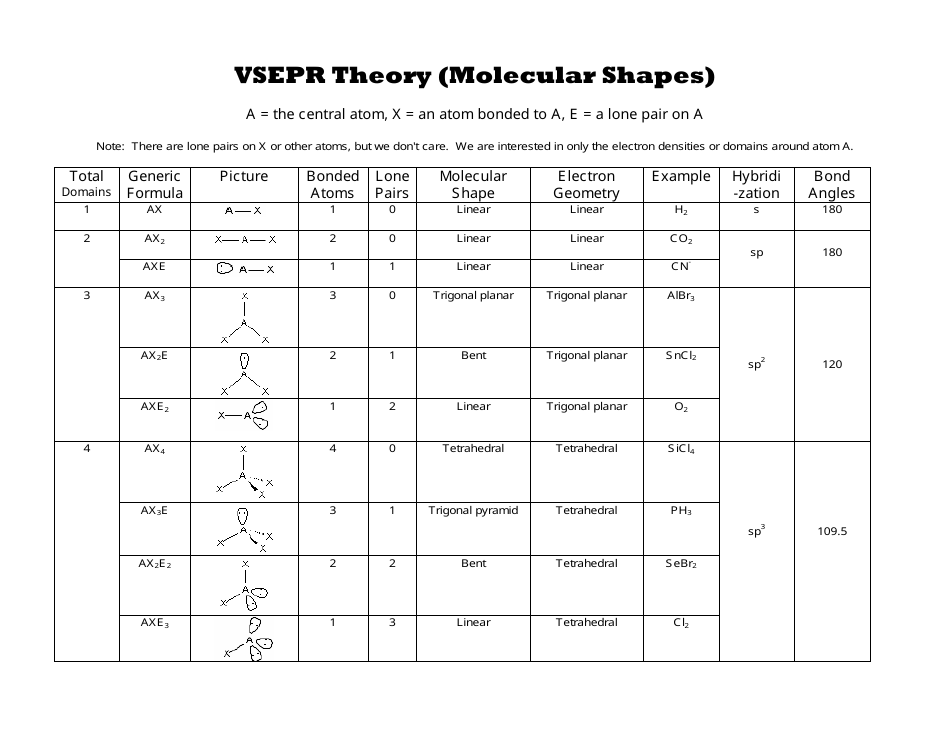
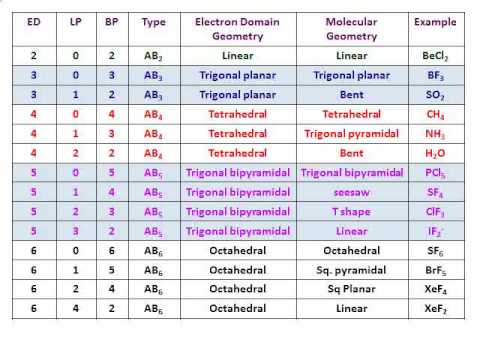
Electron pair geometry chart. Electron Domains Electron-Domain Geometry Predicted Bond Angles Hybridization of Central Atom Molecular Geometry 0 Lone Pair 1 Lone Pair 2 Lone Pair 2 Linear 180º sp Linear 3 Trigonal Planar 120º sp2 Trigonal Planar Bent 4 Tetrahedral 1095º sp3 Tetrahedral Trigonal Pyramidal Bent. In more simple words lone pairs are included in electron geometry and not in molecular geometry. It reflects the electron pairs circling a central atom.
If one s and one p orbital hybridize they form two sp hybrid orbitals. This applies whether they are bonding electrons or non-bonding electrons. Electron Groups Bonding Groups Lone Pairs Electron Geometry Hybridization Molecular Geometry VSEPR class Approximate Bond Angles 5 0 Trigonal Bipyramidal AX 5 4 1 Seesaw AX 4 E 3 2 T-Shaped AX 3 E 2 2 3 Linear AX 2 E 3 180 6 0 Octahedral AX 6 5 1 Square Pyrimidal AX 5 E 4 2.
Name of 3-1 Drawing 3-1 Shape Bond Angle Molecule PH CCI cs STOP Molecular Geometry Lewis Structure. F 2 bonds 1 lone pair. A sample picture of electron-group shape represents the appearance of the molecule.
Molecular Geometry Boundless Chemistry. H 3 bonds 2 lone pairs. This column contains the amount of connected atoms.
If asked for the electron-pair geometry on the central atom we must respond with the electron-pair. In NOCl A is Nitrogen we have one O and one Cl around Nitrogen n 2 and there are two lone electrons ie. You may come across questions asking for the electron geometry as.